Cleaning, Disinfection, and Sterilization are the cornerstones in ensuring safety of Patients in a Hospital settings
Approximately 46.5 million surgical procedures and even more invasive medical procedures—including approximately 5 million gastrointestinal endoscopies—are performed each year. Each procedure involves contact by a medical device or surgical instrument with a patient’s sterile tissue or mucous membranes. A major risk of all such procedures is the introduction of pathogens that can lead to infection. Failure to properly disinfect or sterilize equipment carries not only risk associated with breach of host barriers but also risk for person-to-person transmission (e.g., hepatitis B virus) and transmission of environmental pathogens (e.g., Pseudomonas aeruginosa).
Disinfection and sterilization are essential for ensuring that medical and surgical instruments do not transmit infectious pathogens to patients. Because sterilization of all patient-care items is not necessary, health-care policies must identify, based on the items’ intended use, whether cleaning, disinfection, or sterilization is indicated.


Definition of Terms
Sterilization describes a process that destroys or eliminates all forms of microbial life and is carried out in health-care facilities by physical or chemical methods.
Disinfection describes a process that eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects
Cleaning is the removal of visible soil (e.g., organic, and inorganic material) from objects and surfaces and normally is accomplished manually or mechanically using water with detergents or enzymatic products.
Cleaning
Thorough cleaning is essential before high-level disinfection and sterilization because inorganic and organic materials that remain on the surfaces of instruments interfere with the effectiveness of these processes. As the instruments are used, they are supposed to be immediately washed under running water to remove all dust contents. Post surgeries the blood spillage or adhered protein contents are difficult to remove with water. Enzymes can be used to remove such organic matter.
- To remove protein content -protease enzyme
- To remove carbohydrate/sugar – amylase enzyme
- To remove lipid content- Lipase enzyme
Disinfection
Disinfection describes a process that eliminates many or all pathogenic microorganisms on inanimate objects, except bacterial spores. Disinfection is usually accomplished by the use of liquid chemicals or wet pasteurization in health care settings. The efficacy of disinfection is affected by several factors, each of which may nullify or limit the process’s efficacy.
Some of the factors that affect both disinfection and sterilization efficacy are prior to the cleaning of the object; which includes
- the organic and inorganic load present;
- the type and level of microbial contamination;
- the concentration of and exposure time to the germicide;
- the nature of the object (e.g., crevices, hinges, and lumens);
- the presence of biofilms;
- the temperature and pH of the disinfection process; and,
- in some cases, the relative humidity of the sterilization process (e.g., with ETO).
Sterilization
Most medical and surgical devices used in healthcare facilities are made of materials that are heat stable and therefore undergo heat, primarily steam, sterilization. Sterilization destroys all microorganisms on the surface of an article or in a fluid to prevent disease transmission associated with the use of that item. Medical devices that have contact with sterile body tissues or fluids are considered critical items. These items should be sterile when used because any microbial contamination could result in disease transmission. Such items include surgical instruments, biopsy forceps, and implanted medical devices.
Spaulding’s Classification
Disinfection and sterilization are essential for ensuring that surgical instruments do not transmit infectious pathogens to patients. By definition, disinfection differs from sterilization by its lack of sporicidal property, but this is an oversimplification. A few disinfectants will kill spores with prolonged exposure times (e.g., 3 to 12 hours) and are called chemical sterilants. At similar concentrations but with shorter exposure periods (e.g., 12 minutes for 0.55% ortho-phthalaldehyde) these same disinfectants will kill all microorganisms except for large numbers of bacterial spores and are called high-level disinfectants. Low-level disinfectants may kill most vegetative bacteria, some fungi, and some viruses in a practical period of time (≤10 minutes), whereas intermediate-level disinfectants may be cidal for mycobacteria, vegetative bacteria, most viruses, and most fungi but do not necessarily kill bacterial spores. The germicides differ markedly among themselves primarily in their antimicrobial spectrum and rapidity of action.
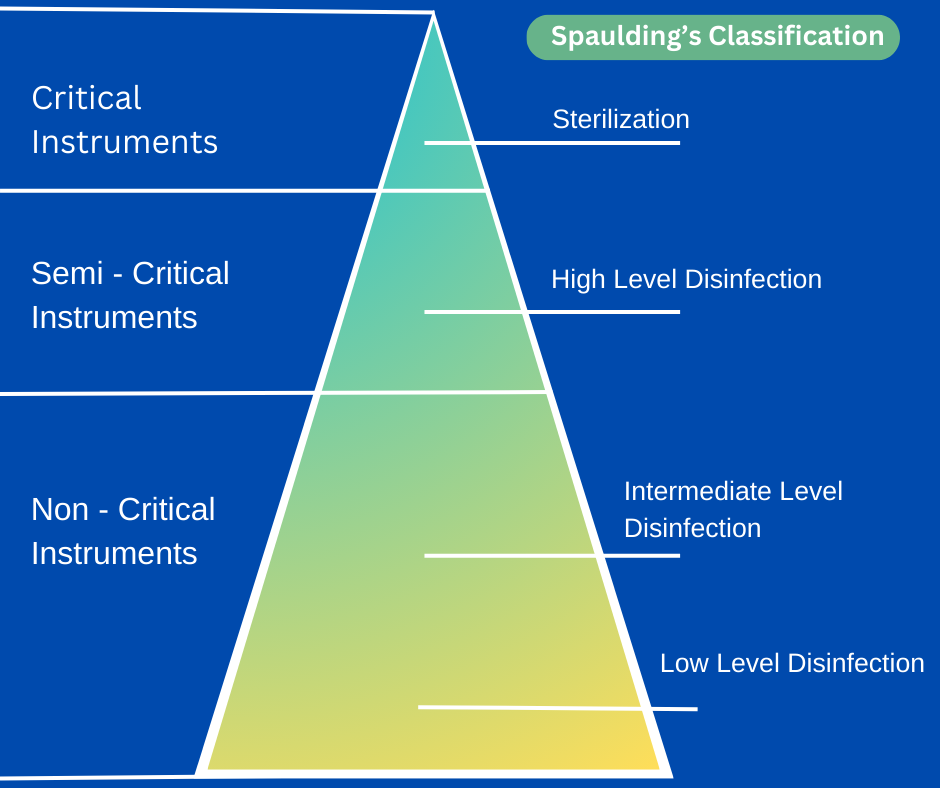
There are several kinds of instruments available in hospitals (medical devices, equipment & surgical materials). About 45 years ago, Earle H. Spaulding devised a rational approach to disinfection and sterilization of patient care items or equipment.
The three categories Spaulding described for instruments used in hospitals are based on the contact or penetration levels of instruments on patient’s body and surroundings. The three categories are critical, semi critical, and noncritical.
Critical Items
These are so called because of the substantial risk for infection if such an item is contaminated with any microorganism, including bacterial spores. Thus, it is critical that objects that enter sterile tissue or the vascular system be sterile because any microbial contamination could result in disease transmission. E.g., surgical instruments, cardiac catheters, implants, pertinent components of the heart- lung oxygenator and the blood compartment of a hemodialyzer. Sterility at the time of use is required for these items.
Semi-Critical Items
These items come in contact with intact mucous membranes, but they do not ordinarily penetrate body surfaces. E.g., Noninvasive flexible and rigid fiberoptic endoscopes, endotracheal tubes, anesthesia breathing circuits, and cystoscopes
Non-Critical Items
These Items are those that either do not ordinarily touch the patient or touch only intact skin. Intact skin acts as an effective barrier to most microorganisms; therefore, the sterility of items that come in contact with intact skin is “not critical.” E.g., crutches, bed boards, blood pressure cuffs, stethoscope, bed-side cupboards, and other medical accessories.
When properly used, disinfection and sterilization can ensure the safe use of invasive and non-invasive medical devices. However, disinfection and sterilization guidelines must be strictly followed.
Reference:
- Mohapatra S. Sterilization and Disinfection. Essentials of Neuroanesthesia. 2017:929–44. doi: 10.1016/B978-0-12-805299-0.00059-2. Epub 2017 Mar 31. PMCID: PMC7158362.
- Centers for Disease Control & Prevention – Cleaning Disinfection & Sterilization Guidelines https://www.cdc.gov/infectioncontrol/guidelines/disinfection/cleaning.html#:~:text=Surgical%20instruments%20should%20be%20presoaked,difficult%2Dto%2Dclean%20instruments.
- Rutala WA, Weber DJ. Disinfection, Sterilization, and Control of Hospital Waste. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 2015:3294–3309.e4. doi: 10.1016/B978-1-4557-4801-3.00301-5. Epub 2014 Oct 31. PMCID: PMC7099662.

